 |

Chemical names:
|
2,6-xylenol or 2,6-dimethylphenol |
| Molecular formula: |
C8H10O |
| Structural formula: |
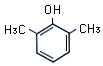 |
2,6-Xylenol is a phenolic compound with CH3
groups adjacent to the OH group. The OH group and para-position
hydrogen atom are chemically reactive.
2,6-Xylenol was first identified in coal tar together with other
phenolic compounds. However, it occurs naturally only in small
amounts, making it difficult to isolate large quantities of
high-quality 2,6-xylenol from coal tar. Recognizing the potential
industrial utility of this compound, researchers throughout
the world spent many years searching for processes by which
it could be synthesized commercially.
Asahi Kasei Corporation began producing 2,6-xylenol commercially
in October 1984, using proprietary technology. Asahi Kasei’s
2,6-xylenol production is performed by Nippon Crenol Co., Ltd.,
a joint venture between Asahi Kasei and Nippon Steel Chemical
Co., Ltd. located at the Asahi Kasei plant complex in Kawasaki.
Asahi Kasei’s production technology enables the production
of 2,6-xylenol and ortho-cresol in the same process
in a fluidized bed reactor, using phenol and methanol as feedstocks.
A catalyst with high selectivity and activity, and leading-edge
plant technology, are used to obtain high product quality.
The basic properties and characteristics of 2,6-xylenol are
shown below. Please inquire for further information.
| Basic properties and characteristics |
| Molecular
formula |
C8H10O |
| Molecular
weight |
122.17 |
| CAS
No. |
576-26-1 |
| Appearance |
White or pale yellow spicular
crystals |
| Odor |
Phenolic odor |
| Density
(solid at 25°C) |
1132 kg/m3 |
| Specific
gravity (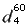 ) ) |
0.982 |
| Melting
point |
45.62  0.01°C
0.01°C |
| Boiling
point (101.3 kPa) |
201.030  0.001°C
0.001°C |
| Vapor
pressure (solid at 25°C) |
8.00 Pa |
|
Constants
in Antoine equation
log10
P = A - B/(T + C)
Where P is vapor pressure (kPa) and
T is temperature (°C)
|
if T
is 4 - 40, then
A = 11.1785  0.029
0.029
B = 3948.27  9
9
C = 273
if T is 144 - 203, then
A = 6.18243  0.079
0.079
B = 1618.523  5.9
5.9
C = 186.482  0.68
0.68
|
| Specific
gravity of vapor (air = 1) |
4.2 |
| Latent
heat of vaporization (101.3 kPa) |
44.522  0.117 kJ/mole
0.117 kJ/mole |
| Heat
of combustion (solid at 25°C) |
4339.85 kJ/mole |
| Heat of
formation |
solid
at 25°C |
237.44 kJ/mole |
| gas at 25°C |
161.84 kJ/mole |
| Critical
temperature |
427.8°C |
| Viscosity |
at 80°C |
0.00102 Pa•s |
| at 150°C |
0.00054 Pa•s |
| pK
of dissociation at 25°C |
10.59 |
| Dipole
moment (D) |
1.410  0.004
0.004 |
| Flash
point (tag closed cup) |
86°C |
| Lower
explosion limit (in air) |
1.04% by volume |
|
| Standard specifications |
| Purity
(excluding water) |
99.5%
min. |
| Water |
0.5%
max. |
| Color
(Hazen) |
APHA
500 max. |
|
|
|
|
 |